ISSN 0253-2778
CN 34-1054/N
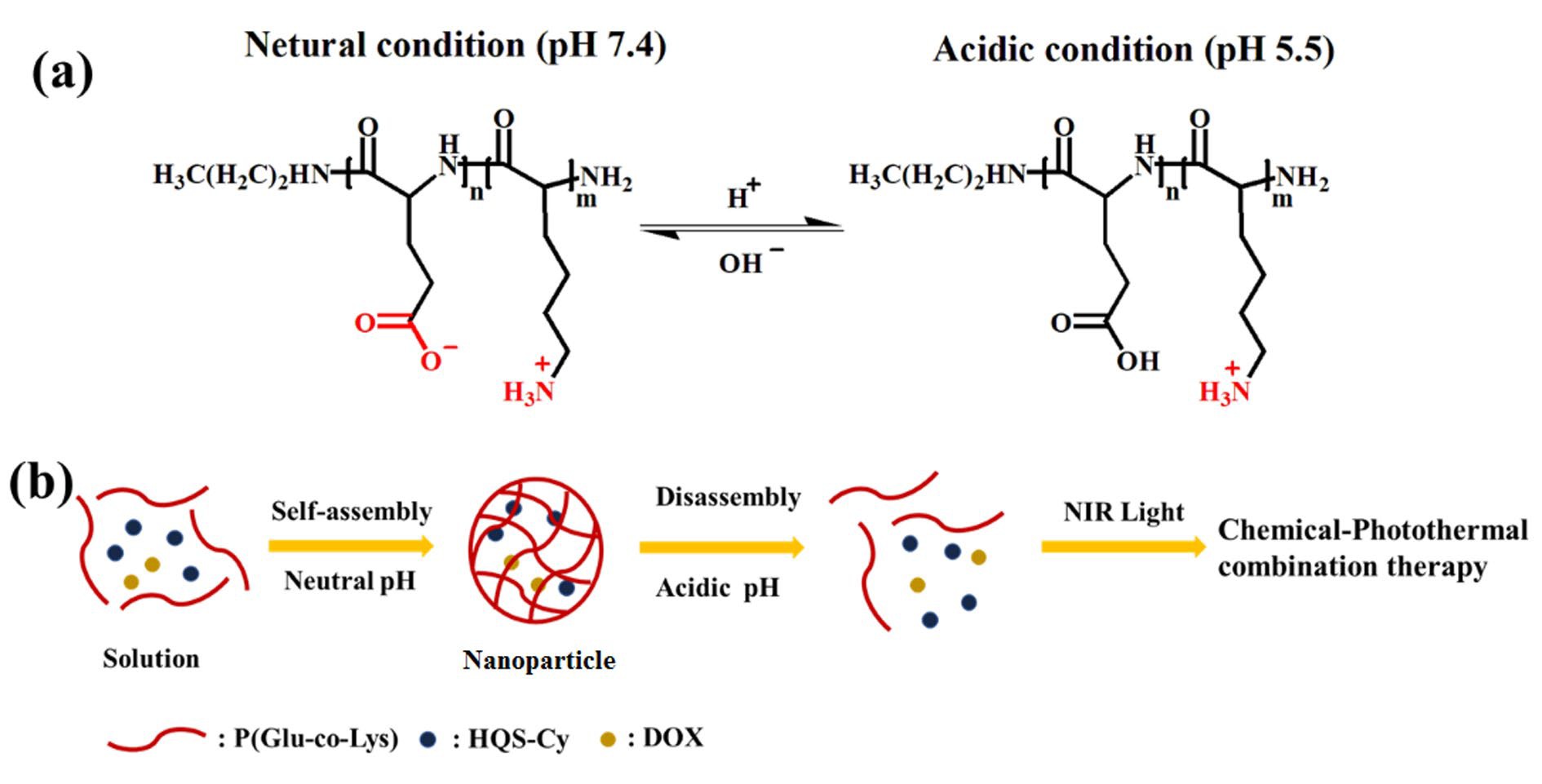
A new smart supramolecular polypeptide copolymer P(Glu-co-Lys) was synthesized by the polymerization of α-amino acids using the N-thiocarboxylic acid anhydride (NTA) method, using the pH dynamic response peptide of L-glutamic acid and L-lysine as a carrier for tumor cells. The drug delivery system activated by external acid can self-assemble (pH 7.4) and disassemble (pH 5.5) under the adjustment of pH to load the drug and control its release. Doxycycline (DOX) and the photothermal reagent hydrophilic quanternary stereo-cyanine (HQS-Cy) were loaded into the peptide copolymer to obtain HQS-Cy/DOX nanoparticles (NPs) for chemo-photothermal therapy. Gentle photothermal heating can enhance the absorption of drugs by cells and enhance the efficacy of chemotherapy. In addition, chemo-photothermal therapy can solve the defect of easy recurrence after single photothermal therapy. The ingenious nanodrug delivery system of HQS-Cy/DOX NPs provides great potential for the improvement of chemo-photothermal therapy and will achieve excellent therapeutic effects in cancer treatment.
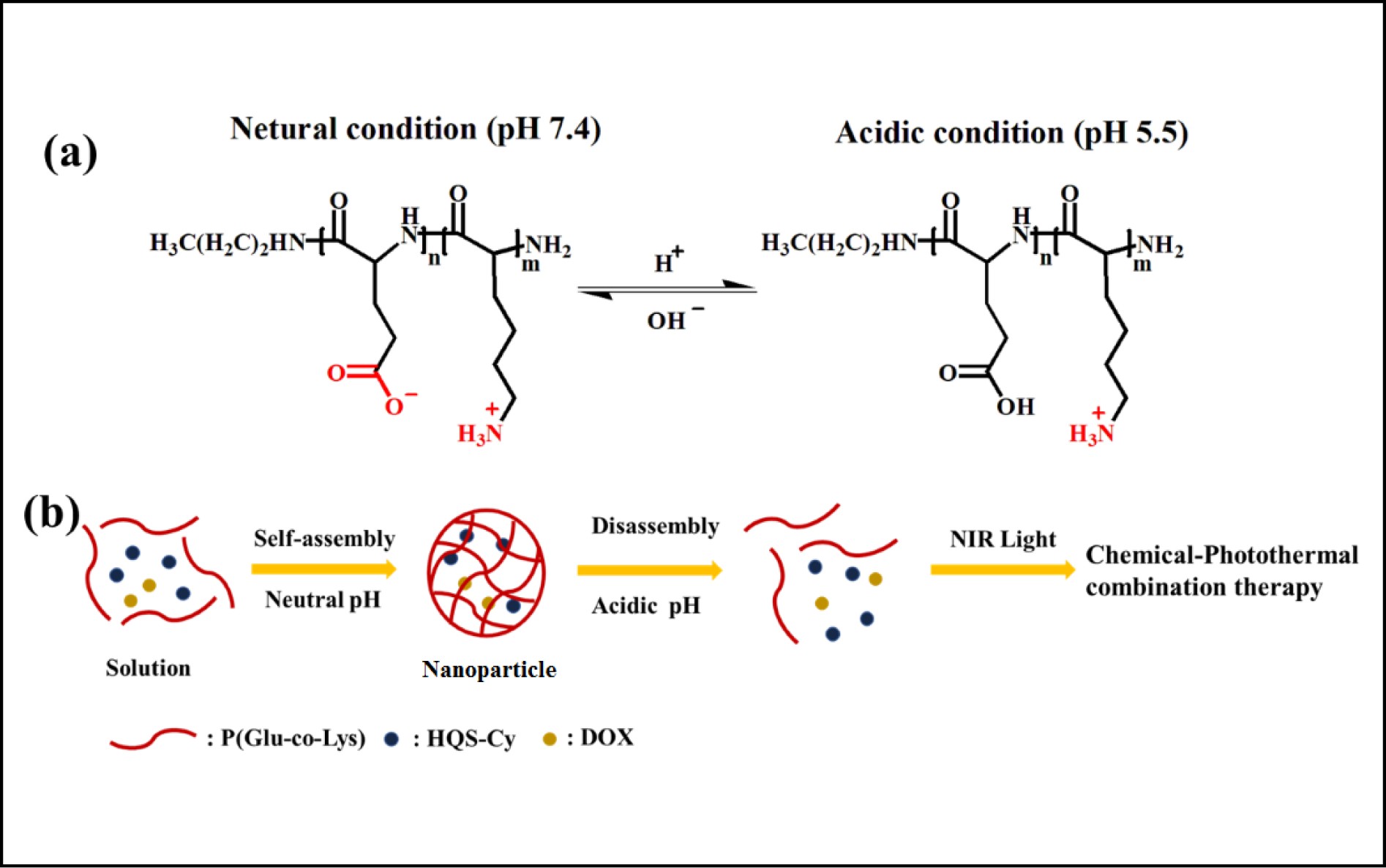
pH-switchable polypeptide nanoparticles for chemo-photothermal therapy.
| [1] |
Fan L, Jin B Q, Zhang S L, et al. Stimuli-free programmable drug release for combination chemo-therapy. Nanoscale, 2016, 8: 12553–12559. DOI: 10.1039/C5NR06305A
|
| [2] |
Suo A L, Qian J M, Zhang Y P, et al. Comb-like amphiphilic polypeptide-based copolymer nanomicelles for co-delivery of doxorubicin and P-gp siRNA into MCF-7 cells. Mat. Sci. Eng. C-Mater., 2016, 62: 564–573. DOI: 10.1016/j.msec.2016.02.007
|
| [3] |
Xu W J, Qian J M, Hou G H, et al. Hyaluronic Acid-functionalized gold nanorods with pH/NIR dual responsive drug release for synergetic targeted photothermal chemotherapy of breast cancer. ACS Appl. Mater. Inter., 2017, 9: 36533–36547. DOI: 10.1021/acsami.7b08700
|
| [4] |
Wang Z Z, Chen Z W, Liu Z, et al. A multi-stimuli responsive gold nanocage-hyaluronic platform for targeted photothermal and chemotherapy. Biomaterials, 2014, 35: 9678–9688. DOI: 10.1016/j.biomaterials.2014.08.013
|
| [5] |
Aubert P, Knott E B. Synthesis of thiazolid-2:5-dione. Nature, 1950, 166: 1039–1040. DOI: 10.1038/1661039b0
|
| [6] |
Higashimura T, Kato H, Suzuoki K, et al. Condensation polymerization of N-dithiocarbonyl alkoxycarbonyl-amino acids. Part I. Synthesis and condensation polymerization of N-dithiocarbonyl ethoxycarbonyl-amino acids. Makromolekul Chem., 1966, 90: 243–248. DOI: 10.1002/macp.1966.020900123
|
| [7] |
Dewey R S, Schoenewaldt E F, Joshua H, et al. Synthesis of peptides in aqueous medium. V. Preparation and use of 2, 5-thiazolidinediones (NTA’s). Use of 13C-H nuclear magnetic resonance signal as internal standard for quantitative studies. J. Am. Chem. Soc., 1968, 90: 3254–3255. DOI: 10.1021/ja01014a059
|
| [8] |
Kricheldorf H R, Bösinger K. Mechanismus der NCA-polymerisation, 3. Über die amin-katalysierte polymerisation von sarkosin-NCA und-NTA. Makromol. Chem., 1976, 177: 1243–1258. DOI: 10.1002/macp.1976.021770502
|
| [9] |
Deming T J. Synthetic polypeptides for biomedical applications. Prog. Polym. Sci., 2007, 32: 858–875. DOI: 10.1016/j.progpolymsci.2007.05.010
|
| [10] |
Choe U J, Sun V Z, Tan J K Y, et al. Self-assembled polypeptide and polypeptide hybrid vesicles: From synthesis to application. In: Deming T, editor. Peptide-Based Materials. Berlin, Heidelberg: Springer, 2011: 117–134.
|
| [11] |
Li J G, Wang T, Wu D L, et al. Stimuli-responsive zwitterionic block copolypeptides: Poly(N-isopropylacrylamide)-block-poly(lysine-co-glutamic acid). Biomacromolecules, 2008, 9: 2670–2676. DOI: 10.1021/bm800394p
|
| [12] |
Rodríguez-Hernández J, Lecommandoux S. Reversible inside-out micellization of pH-responsive and water-soluble vesicles based on polypeptide diblock copolymers. J. Am. Chem. Soc., 2005, 127: 2026–2027. DOI: 10.1021/ja043920g
|
| [13] |
Frisch H, Unsleber J P, Lüdeker D, et al. pH-switchable ampholytic supramolecular copolymers. Angew. Chem. Int. Ed., 2013, 52: 10097–10101. DOI: 10.1002/anie.201303810
|
| [14] |
Behanna H A, Donners J J J M, Gordon A C, et al. Coassembly of amphiphiles with opposite peptide polarities into nanofibers. J. Am. Chem. Soc., 2005, 127: 1193–1200. DOI: 10.1021/ja044863u
|
| [15] |
Wang C, Xu H, Liang C, et al. Iron oxide @ polypyrrole nanoparticles as a multifunctional drug carrier for remotely controlled cancer therapy with synergistic antitumor effect. ACS Nano, 2013, 7: 6782–6795. DOI: 10.1021/nn4017179
|
| [16] |
Liu T, Wang C, Cui W, et al. Combined photothermal and photodynamic therapy delivered by PEGylated MoS2 nanosheets. Nanoscale, 2014, 6: 11219–11225. DOI: 10.1039/C4NR03753G
|
| [17] |
Qian H Y, Cheng Q, Tian Y L, et al. An anti-aggregation NIR-II heptamethine-cyanine dye with a stereo-specific cyanine for imaging-guided photothermal therapy. J. Mater. Chem. B, 2021, 9: 2688–2696. DOI: 10.1039/D1TB00018G
|